We were already anticipating that firm steps were being taken towards the unification of the vaccination calendar and a first agreement has already been reached on basic vaccines and ages of application, although it will not be until 2013 when the complete calendar is unified.
The Ministry of Health has just announced a historic consensus in which for the first time all the autonomous communities have agreed to make effective the basic vaccinations common at the same ages.
This will allow all Spanish children to receive basic vaccines at the same ages and not as it happened (and still happens) that the same vaccine is applied at different ages according to the community of residence.
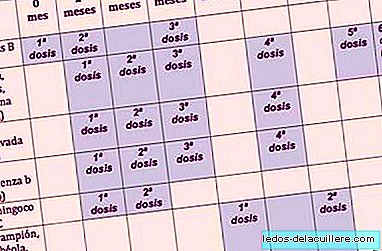
The modifications that have been approved so far have been known, so the basic vaccination calendar in Spain, with the vaccines on which consensus has been reached (they are not all vaccines), it is the one you can see in the box.
Basic Vaccination Calendar
It has reached a first agreement with respect to the age of application of the basic vaccines, although a technical study has been commissioned to unify the complete calendar.
The approved following modifications:
First year of life
- Hepatitis B (HB) at 0, two and six months, with an implementation period of one year.
- Meningococcus C (MenC) at two and four months, with an implementation period of one year.
Second year of life
- DTPa-VPI-Hib, fourth booster dose at 18 months, with an implantation period of one year
- Viral Triple (SRP), first dose at 12 months, with an implantation period of one year.
Three to six years
- DTpa, fifth booster dose at six years, with an implantation period of one year.
- Viral Triple (SRP), second dose in the age range of three to four years, with an implantation period of three years.
10 to 16 years old
- Td, sixth booster dose at 14 years, with an implantation period of one year.
Vaccines that generate conflict

At the moment it is a calendar of basic vaccines with their respective ages of application. So far there is agreement.
However on the calendar not listed other vaccines recommended by the Spanish Association of Pediatrics such as chickenpox vaccine, recommended at 12-15 months and at 2-3 years, the rotavirus, recommended between 2 and 6 months of age, the pneumococcus, recommended at 2,4,6 and 12-15 months, and the vaccine human papilloma virus Recommended between 11 and 14 years.
Now the most difficult thing will be to bring positions on these vaccines, some of them financed only by some communities such as pneumococcus in Madrid and Galicia, or chicken pox in Madrid, Navarra, Ceuta and Melilla.
They are expensive vaccines that the communities that have them implemented refuse to withdraw, and, although it would be desirable, the rest of the communities are reluctant to include.
They are also not included in the single basic calendar the vaccines recommended for risk groups such as seasonal flu and Hepatitis A.
Single Health Card
Another novelty that will be launched is a single health card.
It will begin broadcasting at the end of 2012 and will allow patients to receive healthcare in any part of the Spanish territory, even if they are in an autonomous community other than their own.
One last step
We will already know how the second part of the agreement is then resolved with respect to the vaccines that generate the most conflict.
So far, the ages of application of basic vaccines, which is a first step towards configuring the unique vaccination calendar in Spain.












